E.A. Decaf
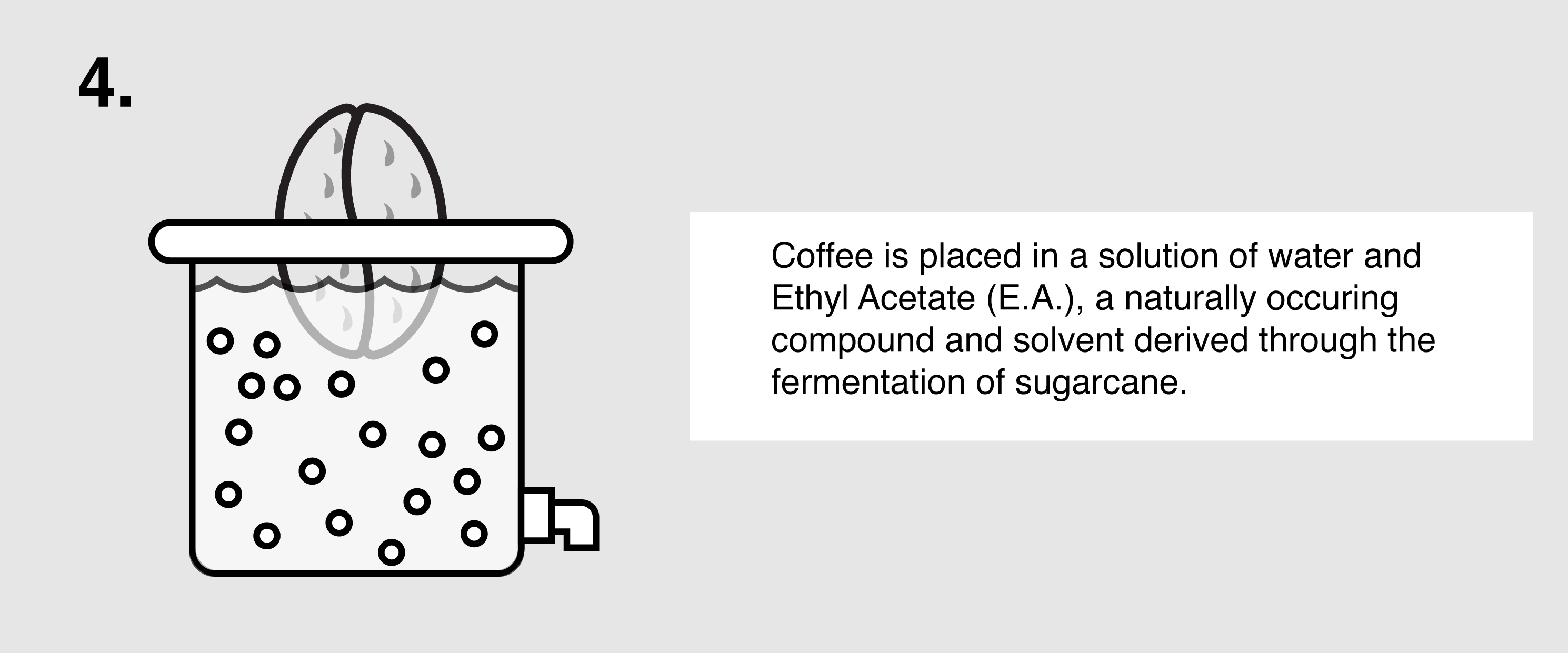
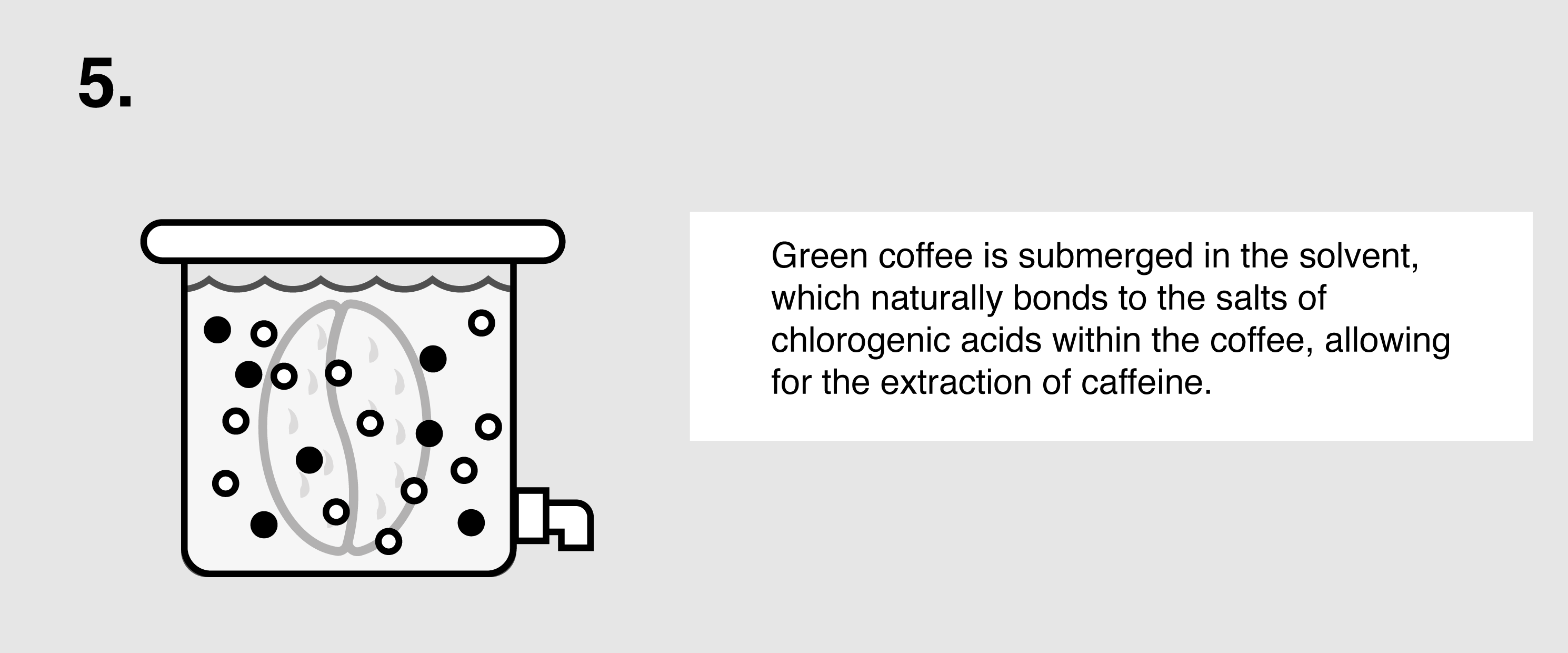
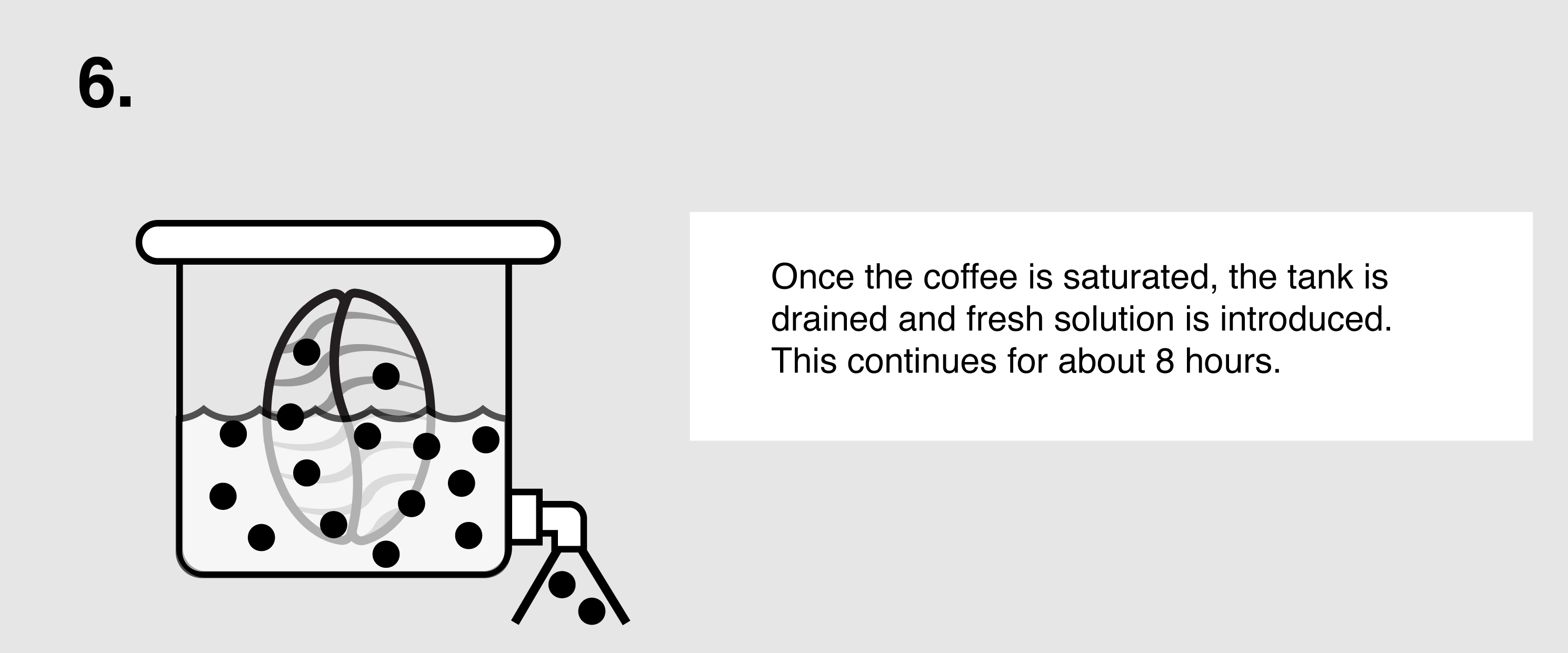
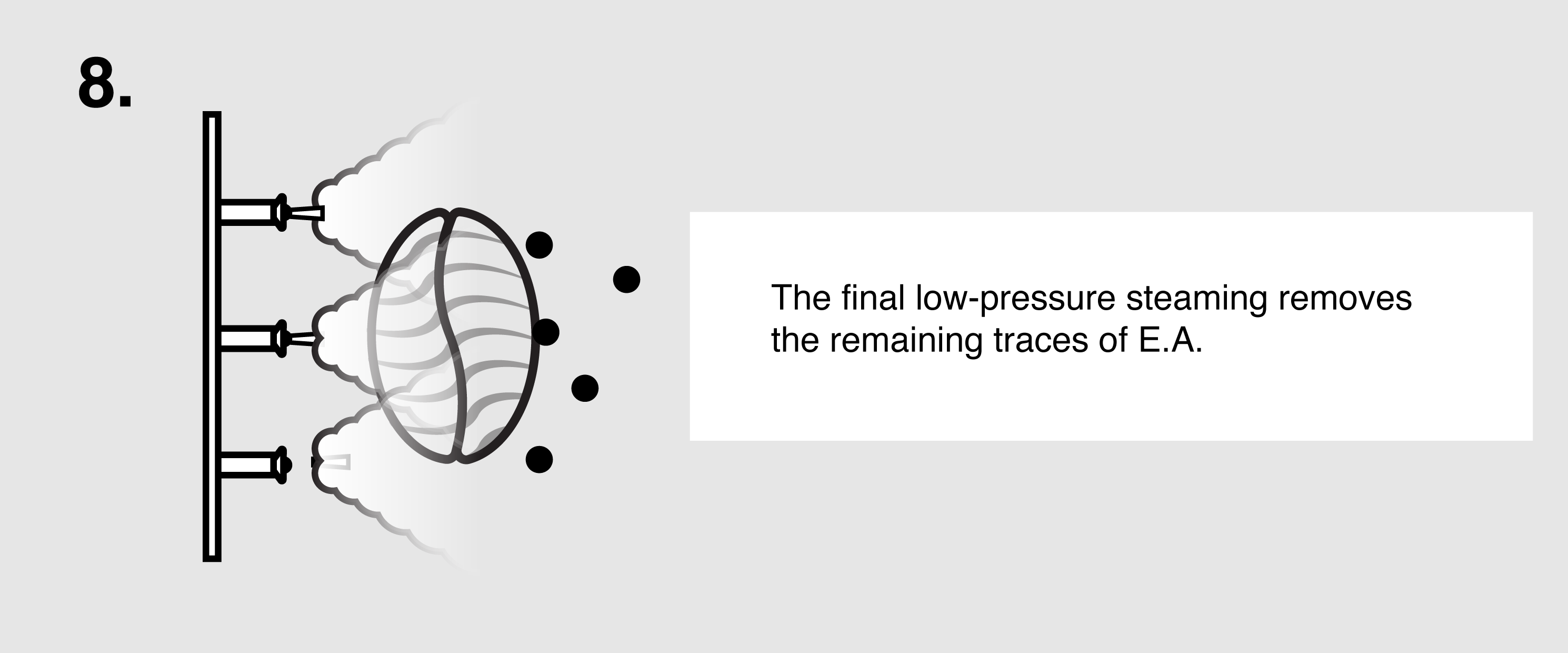
Ethyl Acetate (E.A.) – This naturally occurring ester (present in bananas as well as a by-product of fermented sugars) can be isolated and used as a solvent to bond with and remove caffeine from green coffee. First, the coffee is sorted and steamed for 30 minutes under low pressure in order to open the coffee seeds’ pores and prepare them for decaffeination. The coffee is placed in a solution of both water and ethyl acetate, where the E.A. will begin to bond with the salts of chlorogenic acids inside the seeds. The tank will be drained and re-filled over the course of eight hours until the caffeine is no longer detected. The seeds are steamed once more to remove the ethyl acetate traces, though E.A. is only harmful to humans in very high quantities (400 parts per million or more). The coffee is then dried and polished for export.

Specs
Our E.A. Decaf offerings are processed with a natural ethyl acetate produced from esterification of ethyl alcohol and natural acetic acid. It is produced from sugar-cane carbohydrates by fermentation with Saccharomyces cerevisiae. Natural acetic acid is extracted and purified from vinegar, which is produced by means of aerobic fermentation of ethyl alcohol with the
microorganism Acetobacter aceti.
This product is fit for human consumption and is kosher, halal and Good Manufacturing Practices–certified. Natural ethyl acetate is produced under the Food Safety BRC v7 standards, Quality ISO9001 standards, Health and Safety at Work OHSAS18001 standards, and Supply Chain Assurance ISO28001 standards.
None of the mentioned microorganisms have been genetically modified, guaranteeing natural ethyl acetate is non-GMO according to EC 1829 and 1830/2003 regulations.

The E.A. Decaf Process
Ethyl acetate (E.A.) decaffeination is a natural process that maintains the integrity of green-coffee flavor.
- Ethyl acetate occurs naturally in many fruits, which is why this method is often referred to as natural decaffeination.
- This method requires a thorough steaming of the beans until they swell. An ethyl acetate aqueous solution is used to wash the swollen beans repeatedly.
- Ethyl acetate is a polar molecule, which makes it a good solvent for capturing the polar caffeine molecules from the coffee beans, since “like dissolves like.” The caffeine molecules bond to the ethyl acetate molecules and migrate through the cell membranes of cells of the beans.
Click to download a PDF of our E.A. Decaf diagram, or view the slideshow below to walk through the process step-by-step.